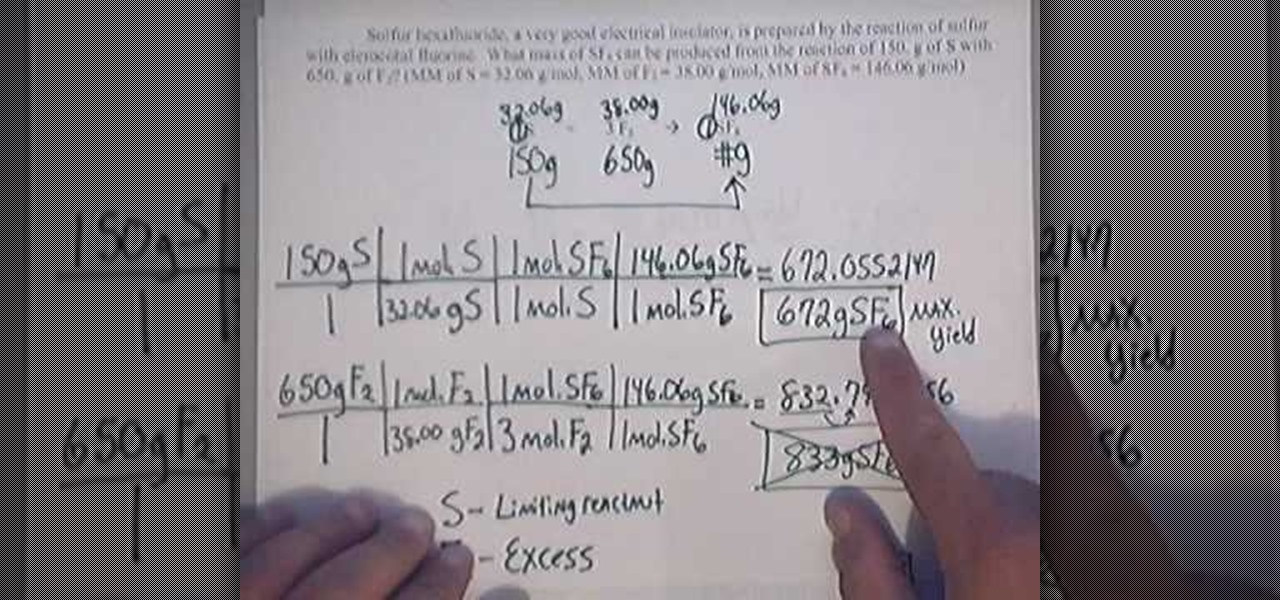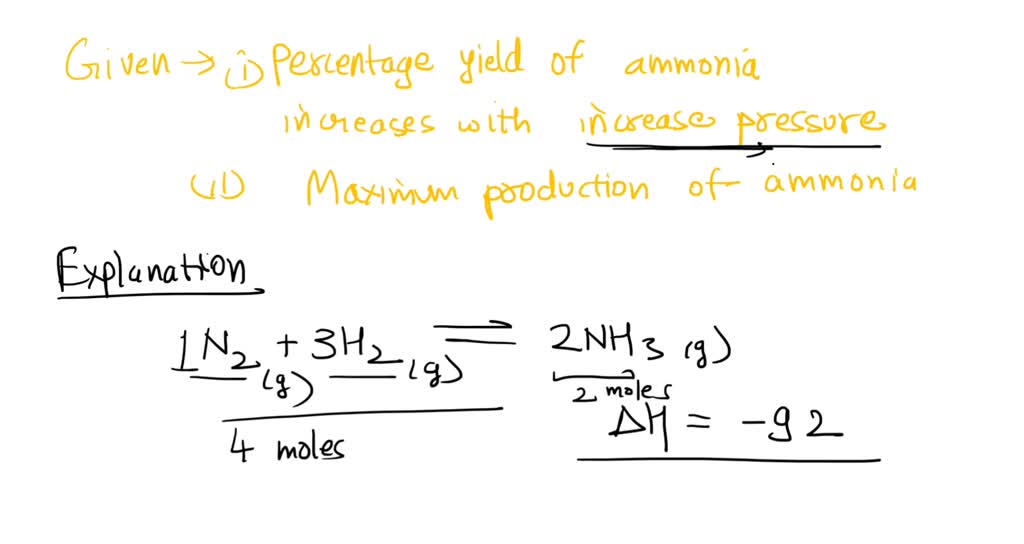Unbelievable Info About How To Increase Percent Yield

This means we will get 2 moles of the product too.
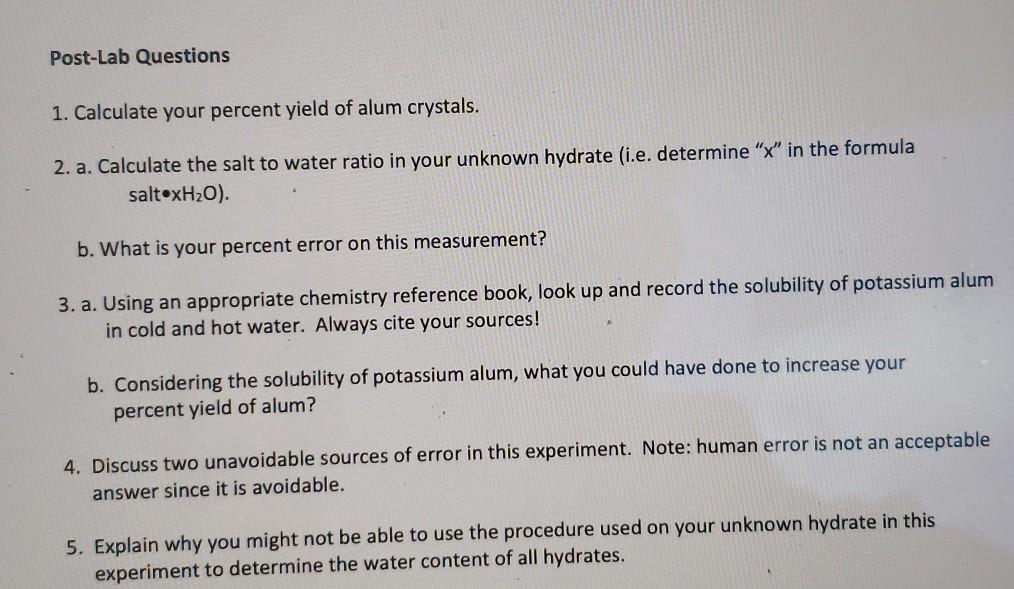
How to increase percent yield. Actual yield is the amount of product obtained from a chemical reaction. And with what substrate do you react it? * mix different amounts of the two chemicals.
As we mentioned, the third step is to subtract your total ending value from the initial investment. What does the percent yield tell you about your results? Simple things to try :
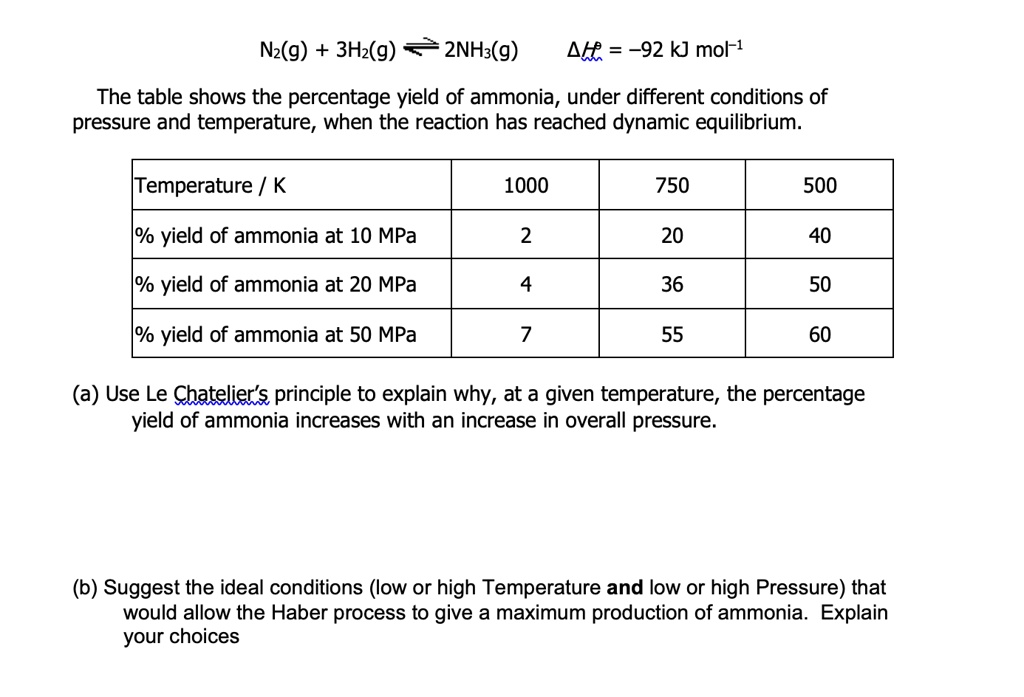
The equation for percent yield is: How to work with thiols. * change reaction conditions (temprature or pressure) * use a catalyst.
Theoretical yield is the amount of product obtained from the stoichiometric or balanced equation, using the limiting reactant to determine product. Suppose for this reaction, we have 2 moles of reactant. Alternatively, one of your reagents was limiting, and caused a reduced yield.
Tips for flash column chromatography. I have seen many grignard reactions performed on the large scale…and in the. Veratrole was the limiting reagent and it was.
Now, you have to divide the gain $100 by the total ending value of $1,100 to get 0.09. [percent yield] = ( [actual yield] / [theoretical yield] ) * 100% here’s a quick example: Well, what grignard are you making?